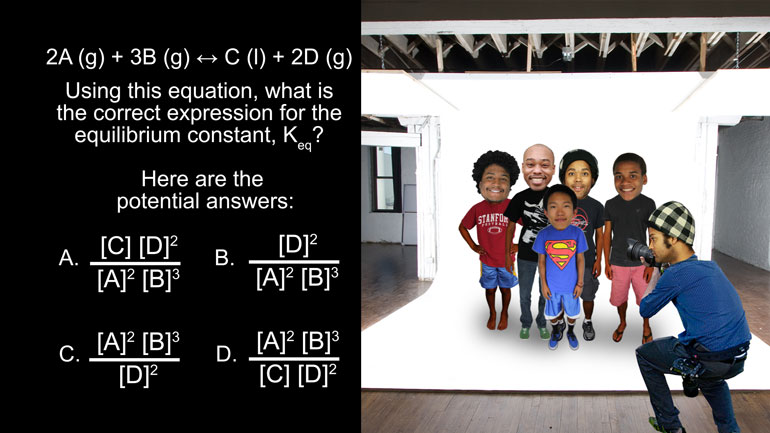
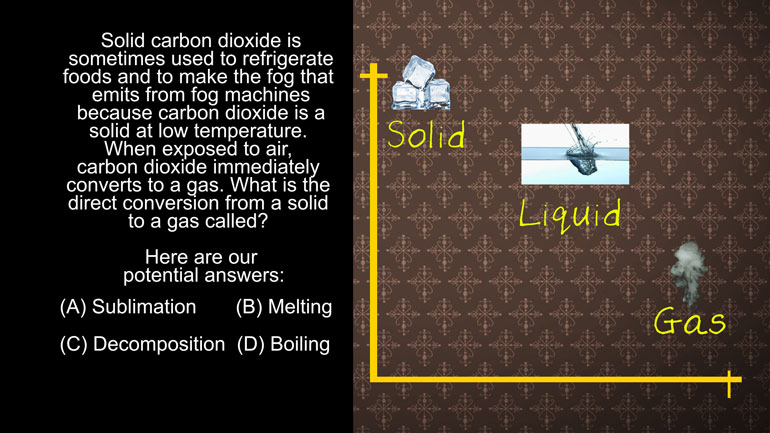
ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 45 videos
AP Chemistry 3.5 Chemical Reaction Rates. How much 85Kr will we need to collect?
Want to pull an Oliver Twist and ask us for more? We've gotcha covered. Head over to /video/subjects/test-prep/ap/ap-chemistry/ for more AP Ch...
AP Chemistry 1.3 Forming and Breaking Bonds. Which of the following ions are spectator ions?
AP Chemistry 3.1 Chemical Reaction Rates 3 Views
Share It!
Description:
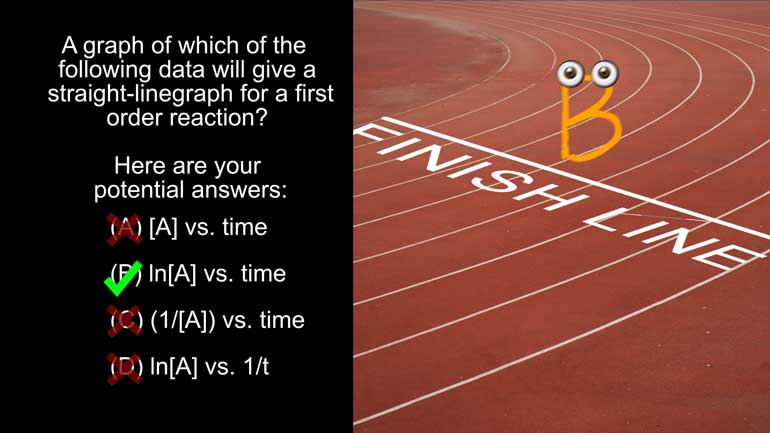
AP Chemistry 3.1 Chemical Reaction Rates. A graph of which of the following data will give a straight line graph for a first order reaction?
Transcript
- 00:03
Here’s your Shmoop du jour, brought to you by lines, helping scientists get dates since
- 00:08
1811. [Boy and girl looking at paintings]
- 00:09
Because what's dating without a little…chemistry?
- 00:12
Alright, here’s today’s question:
- 00:14
A graph of which of the following data will give a straight-line graph for a first order
Full Transcript
- 00:18
reaction?
- 00:19
And here are your potential answers: All right, so we know that for a first order
- 00:25
reaction, the rate is proportional to the concentration of the reactant. [First order reaction equation]
- 00:29
That means that as the reaction proceeds, the concentration of the reactant decreases
- 00:33
more and more slowly.
- 00:35
To be a little more specific, the concentration of [A] decays exponentially.
- 00:40
Just like your concentration in the math class you have right before lunch. [Students in math class]
- 00:44
If your eyes are in proper working order, you can tell by looking at the graph that
- 00:47
this is not a straight line. [Graph with a sloping line]
- 00:49
If it looks like one to you, we highly recommend a quick visit to the optometrist. [Girl conducting an eye-exam]
- 00:52
Anyway, since this is a graph of the concentration of A versus time, we can eliminate A).
- 00:58
Options C) and D) could be right if the reaction orders were different.
- 01:01
But, unfortunately, teachers don’t give credit for potential correctness. [Answers C and D crossed out]
- 01:05
So that means that a graph of B would give you a straight line for a first order reaction.
- 01:10
For first-order reactions, the graph of the natural log of the concentration of the reactant
- 01:15
versus time will be a straight line.
- 01:18
So B will take you straight to the finish line. [B crossing the finish line on a running track]
- 01:21
We’d tell you another chemistry pick up line, but all the good ones argon. [Periodic table and Argon asks to call me]
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
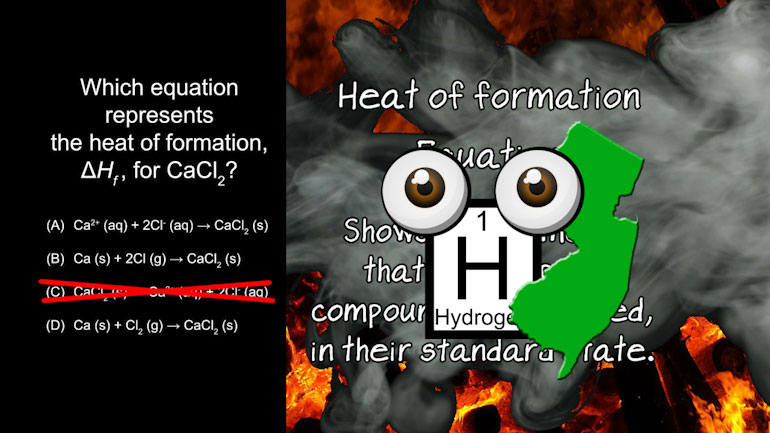
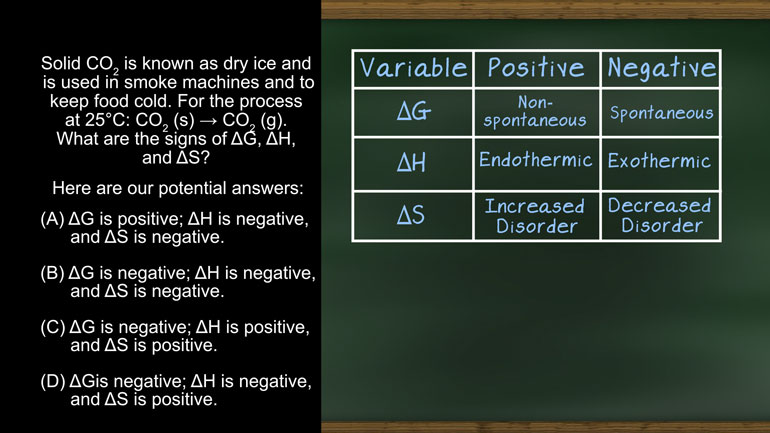
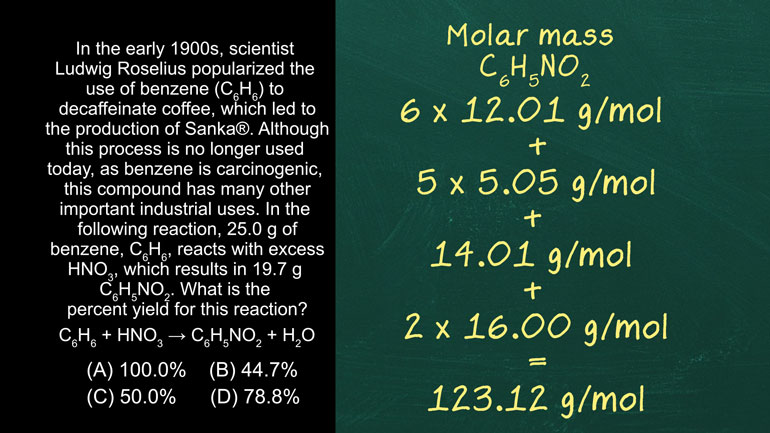
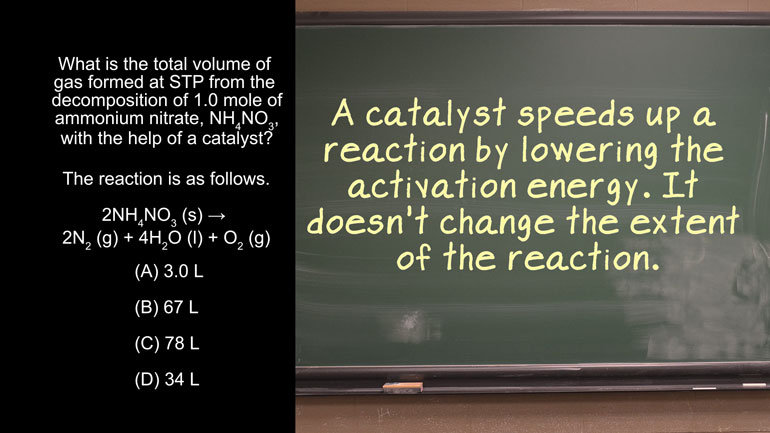
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?