ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
ACT Science: Research Summary Passage Drill 2, Problem 1. Why do you think that the filter paper will not remove the salt from the water?
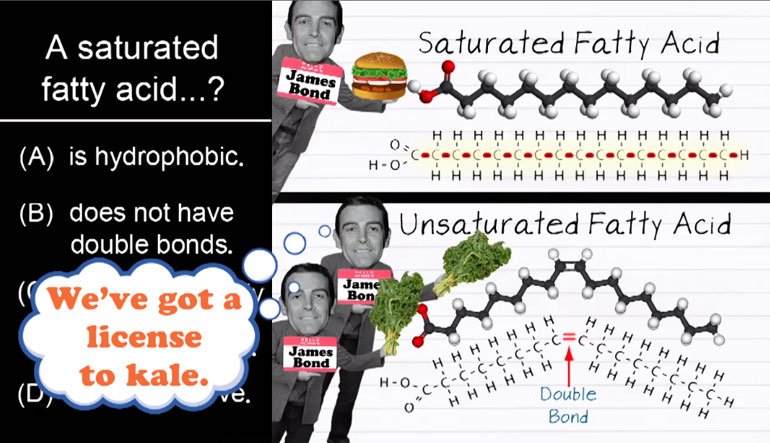
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
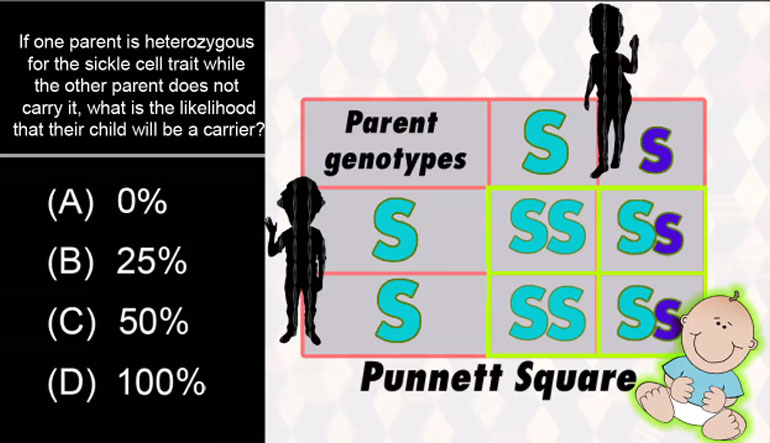
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Chemistry 2.5 Laws of Thermodynamics 8 Views
Share It!
Description:
AP Chemistry 2.5 Laws of Thermodynamics. Which of the following is true for a reaction at equilibrium?
Transcript
- 00:04
And here's your Shmoop du jour, brought to you by equilibrium, the human rights movement [People protesting outside a library]
- 00:08
that insists librarians should be treated equally.
- 00:12
Okay, here's our question.
- 00:14
Which of the following is true for a reaction at equilibrium?
- 00:19
And here are your potential answers.
Full Transcript
- 00:20
Okay, don’t get all “one with nature” on us here…we’re talking about molecular [Boy balancing books on his head]
- 00:26
equilibrium here.
- 00:28
That is, the point at which the forward rate of a reversible chemical reaction equals the
- 00:33
reverse rate. [Equilibrium of reaction depicted on a graph]
- 00:35
The concentrations of reactants and products don’t change, even though reactions are
- 00:38
still taking place.
- 00:41
So A and B make C and D at the same rate that C and D make A and B.
- 00:47
You thinking what we’re thinking? [Two guys jumping up and down]
- 00:48
Great, let's say it together.
- 00:50
3, 2, 1…Bananas!
- 00:53
…Oh.
- 00:54
Or example time.
- 00:55
That's good, too.
- 00:56
Okay, imagine that there’s a team of mathletes who decide that they should socialize more. [Mathletes socialising with each other]
- 01:03
So they schedule a hang-out sesh with the science Olympiad team studying next door.
- 01:07
We're sensing this is going to end in a rager. [Mathletes and Science Olympians together]
- 01:10
…Just us?
- 01:11
At some point, the students are split among the rooms, and the rate at which a mathlete
- 01:15
leaves his room is the same as the rate at which a different mathlete enters. [Mathletes leaving and entering the rooms]
- 01:21
The same is true for the science Olympians.
- 01:23
This smarty-swap can be thought of as our “reaction.”
- 01:27
The relative “concentration” of mathletes and science Olympians remains the same, despite
- 01:33
the fact that there’s still movement and different individuals might be present at [Olympians and Mathletes interchanging between rooms]
- 01:36
any given time.
- 01:38
And here we have achieved the ultimate nerdy equilibrium.
- 01:41
Hm.
- 01:42
Really thought it would end in a rager.
- 01:44
So let’s take another look at our question. [Boy using binoculars]
- 01:47
What do all these variables mean?
- 01:49
Well, DeltaH - or change in enthalpy - tells us if a reaction absorbs or releases
- 01:55
heat.
- 01:56
DeltaG, or the change in Gibbs free energy, tells us if a reaction is spontaneous
- 02:03
or non-spontaneous.
- 02:05
And DeltaS, or the change in entropy, tells us if a reaction leads to more or less [Delta H, G and S definitions]
- 02:10
disorder.
- 02:11
Of the science-y kind, not the mental kind. [Boy spinning a basketball on his finger]
- 02:14
Though too much chemistry will give the best of us anxiety.
- 02:17
Option A just means the reaction releases heat. [Girl cooking marshmallows on a fire]
- 02:19
It doesn’t tell us anything about equilibrium.
- 02:22
Option C tells us that there is no entropy change.
- 02:25
This just means that the degree of disorder remains the same.
- 02:29
Option D implies that there is no heat absorbed or released from the reaction.
- 02:32
All right, Option B. Not to Obi Wan you, but you’re our only hope. [Obi Wan appears and prods option B]
- 02:38
This option is all about spontaneity.
- 02:40
We know that if DeltaG<0, the reaction is spontaneous, and if DeltaG>0, the reaction is not spontaneous.
- 02:48
But what if DeltaG equals zero? [Boys walking a tight rope together]
- 02:50
This means the reaction is at equilibrium, and forward and reverse rates are the same.
- 02:54
So B is the right answer.
- 02:56
And there you have it folks, equilibrium.
- 02:57
Now let’s check on our smarty-pants party before somebody blows up a Bunsen burner. [Olympians and Mathletes socialising together in classrooms]
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
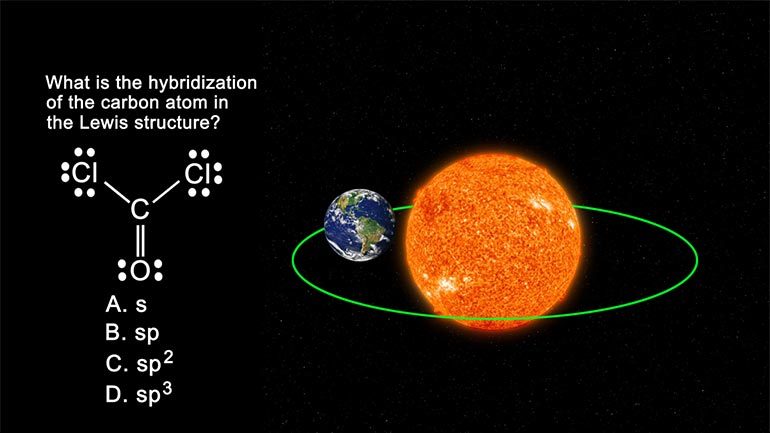
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry DBQ/Free Response. Perform the following calculations.