ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Physics 2: 1.5 Waves 2 Views
Share It!
Description:
AP Physics 2: 1.5 Waves. Which of the following explains why quantum mechanics is only applicable at the particle level?
Transcript
- 00:00
Thank you We sneak and here's your shmoop toos your
- 00:05
brought to you by quantum mechanics And we're not talking
- 00:08
about the tiny guys who can fix a car different
- 00:12
kind of a camp All right which of the following
- 00:14
explains why quantum mechanics is applicability only at the particle
Full Transcript
- 00:18
level Select two answers and here's some choices And we're
- 00:24
done reading Yeah all right Quantum physics is well weird
- 00:29
like really weird Like what the heck is going on
- 00:31
in the universe Weird ever hear someone say you can't
- 00:35
be two places at once Well quantum physics has shown
- 00:39
that some particles can in fact be in two places
- 00:42
at one time and the act of observing a particle
- 00:45
affect how it moves That's right Just looking at something
- 00:48
can determine how that's something acts A lot of these
- 00:51
ideas came from the work of louis debra a french
- 00:54
brainiac who did a lot of work on this stuff
- 00:56
In the nineteen twenties He had a theory that everything
- 00:59
could be looked at as own wave No he wasn't
- 01:02
a surfer at least not that we know of He
- 01:05
was just trying to make sense of the weirdness of
- 01:07
quantum mechanics One thing that's not weird about quantum mechanics
- 01:10
though is the fact that they still inhabit the same
- 01:13
universe as the rest of us way all live in
- 01:16
yellowstone so they have to deal with the same constance
- 01:20
is we do Constance don't change on a subatomic level
- 01:24
because they're a constant so is not one Of the
- 01:27
right answers they're crossing dubray theorized that any moving object
- 01:32
could be observed as a wave Well if you run
- 01:36
around the block you too can be a wave We'll
- 01:39
stay here on the couch though sounds like a lot
- 01:40
of work to be a wave Objects that are human
- 01:43
size have a very tiny wavelength so tiny that it
- 01:47
really doesn't mean anything smaller the object however the larger
- 01:51
the wavelength and you have to get down pretty small
- 01:53
for the observed wave to make a real difference in
- 01:56
how an object interacts with other fields and particles So
- 01:59
answer b is one of the correct choice right there
- 02:02
that's correct and this whole wave particle duality where a
- 02:05
seemingly solid particle can behave like a wave is only
- 02:09
observable at the scale of adam's or even smaller subatomic
- 02:13
particles After all if we run around the block no
- 02:15
one will say look at that wave go They'll say
- 02:19
are you dying Should we call an ambulance And we're
- 02:22
also saying that sea is the other correct answer but
- 02:25
just because it can only be observed on a very
- 02:27
small scale doesn't mean we couldn't calculate the wavelength of
- 02:30
a larger object Wait maybe some crazy number like two
- 02:35
to the negative one thousand but it can definitely be
- 02:38
calculated So d is incorrect Also yeah quantum mechanics air
- 02:43
counterintuitive in a lot of ways so they can be
- 02:44
pretty confusing It's hard to think of A building is
- 02:47
acting like a wave and it's hard to envision something
- 02:50
as tiny as an electron period But it's pretty cool
- 02:53
stuff plus of anyone ever is annoying us While we
- 02:56
could just start explaining quantum physics to him usually they'll
- 02:59
get so confused They'll just act like a wave on 00:03:02.223 --> [endTime] flow away from us
Up Next
Related Videos
AP Physics 2: 1.1 Properties of Objects and Systems. What is the magnitude and direction of the conventional current in this wire?
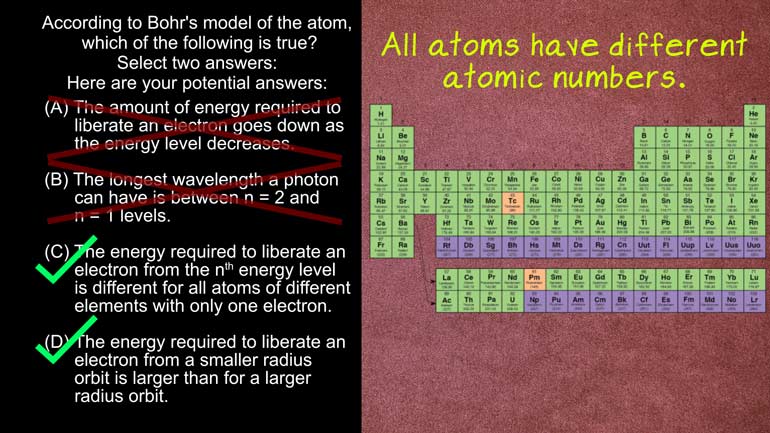
AP Physics 2: 1.5 Properties of Objects and Systems. According to the Bohr's model of the atom, which of the following are true?
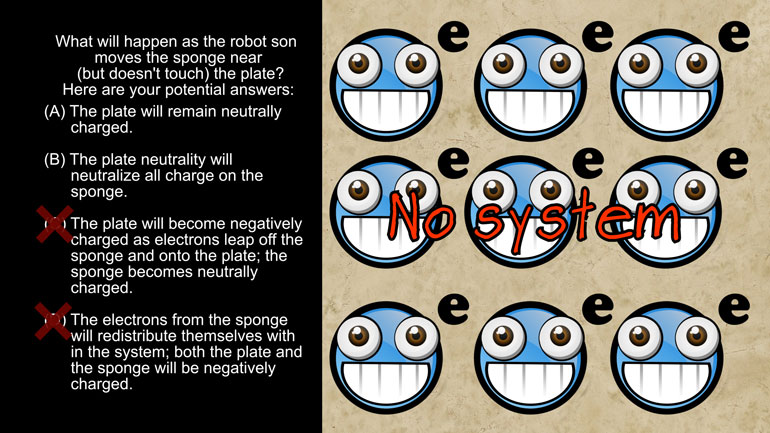
AP Physics 2: 2.2 Properties of Objects and Systems. What will happen as the robot son moves the sponge near (but doesn't touch) the plate?
AP Physics 2: 2.4 Properties of Objects and Systems. How could you show the carnival barker an emission spectrum?